前 言
非酒精性脂肪性肝炎(NASH)是一种与肥胖、血脂异常、2型糖尿病和代谢综合征密切相关的疾病,可能会发展为肝硬化、终末期肝病甚至肝癌。据美国肝脏基金会统计数据显示,截至2023年8月,美国成年人中有5%的NASH患者(而NAFLD患者高达25%),且FDA尚未批准任何用于NASH临床治疗的药物。由于NASH发病机制复杂,药物研发道路曲折漫长,主要集中FASN、THR-β、NR1H4/FXR、GLP-1R、PPARA、FGF19、FGF21、ACLY等靶点。
01 NASH基本概念
非酒精性脂肪性肝病(NAFLD)是指除酒精和其他明确损肝因素外,肝细胞内脂肪过度沉积或代谢紊乱引起的慢性肝病,包括单纯性脂肪肝(NAFL)、非酒精性脂肪性肝炎(NASH)、肝纤维化及肝硬化。2022年9月,柳叶刀子刊发表的一项针对全球NAFLD流行病学系统性回顾和荟萃分析显示,全球成人NAFLD患病率约为32.4%。换句话说,全球平均每3个人中就有1人罹患NAFLD。
非酒精性脂肪性肝炎(NASH)是以脂肪在肝脏的过度积累和炎症为特征,可由多种因素导致。大约有15%-25%的NAFLD患者会发展为NASH。NASH一旦发展为晚期,就会引起肝细胞增殖丧失及再生能力下降,进而引发炎症和肝纤维化,并最终可能导致肝硬化、肝功能衰竭,甚至发展为肝癌。
02 NASH治疗靶点研究
对NASH生理和病理机制进行全面的研究,寻找潜在的治疗靶点和生物标志物,对开发有效的干预措施和治疗方案具有重要意义。目前研究表明,FASN、THR-β、NR1H4/FXR、GLP-1R、PPARA、FGF19、FGF21、ACLY等靶点的在体内的失衡与NASH紧密相关。
根据Evaluate Pharm和Frost & Sullivan的评估,到2025年全球NASH药物市场预计将达到350-400亿美元。Madrigal公司的THR-β激动剂(Resmetirom)治疗NASH的III期临床达到主要终点和关键次要终点,可能会成为首款获FDA批准上市的NASH药物。Akero公布的FGF21类似物(Efruxifermin)结果显示,在IIb期临床试验中可改善41%受试者肝纤维化程度,刷新了NASH新药有效性数据。还有今年在减肥领域大火的Retatrutide(GIP/GLP-1/GCG三受体激动剂)在NASH方面的II期临床数据让人感到惊讶,在98名NAFLD患者中肝脏脂肪显著减少,且NASH相关生物标志物显著改善。
|-----------------|---------------------------------|----------------------------------------------------------------------|-------------------|
| NASH相关治疗靶点与在研药物 ||||
| 靶点 | 作用机制 | 在研药物 | 药理机制 |
| FASN | 失调导致脂肪变性,影响脂代谢,是NASH发生病变的一个重要标志 | FASN抑制剂 | 减少肝脏脂肪积累并减轻炎症 |
| THR-β | 信号传导失调破坏代谢平衡,导致脂肪积累和发生炎症 | THR-β激动剂(Resmetirom) | 调控代谢稳态并改善脂肪变性 |
| NR1H4/FXR | 失调导致胆汁酸介导的肝损伤及炎症反应 | NR1H4/FXR激动剂(Tropifexor、Nidufexor) | 调节胆汁酸代谢和炎症反应 |
| GLP-1R | 功能障碍可能导致胰岛素抗性及脂肪变性 | GLP-1R激动剂(Semaglutide、Liraglutide) | 提高胰岛素敏感性和减少肝脏脂肪变性 |
| PPARA | 信号传导改变导致脂肪代谢受损和加剧炎症 | PPARA激动剂 | 改善脂质代谢和炎症 |
| FGF19、FGF21 | 参与NASH病变肝胆汁酸调节和能量代谢 | FGF19类似物(Aldafermin)、FGF21类似物(Efruxifermin)、FGF21受体激动剂(Pegbelfermin) | 减少脂肪变性和改善肝酶活性 |
| ACLY | 促进脂肪积累 | ACLY抑制剂(Bempedoic) | 预防脂肪肝变性 |
来源:参考文献1-15
03 NASH靶点在研究中的应用
重组靶点蛋白已用于NASH及其他疾病的药物发现和开发研究。Guo团队利用重组人GLP-1受体蛋白(货号:13944-H02H,义翘神州)发现并表征了一种新型GLP-1类似物Ecnoglutide。Qiao团队使用FGF19重组蛋白(货号:12226-HNAE,义翘神州)处理HepG2细胞,证实FGFR4介导FRS2α-ERK通路,为靶向FGFR4治疗肝细胞癌提供理论证据。Hu团队利用FGF19重组蛋白(义翘神州)进行细胞增殖和侵袭实验,发现在晚期浆液性卵巢癌中,发现FGF19-FGFR4信号通路可促进卵巢癌增殖和侵袭。[a1] Lee团队将人FGF21重组蛋白(义翘神州)注射到高血糖小鼠模型,发现FOXO1抑制剂和FGF21联合治疗可提高胰岛素敏感性。
用于表面等离子体共振SPR检测

结果显示GLP-1肽类似物(M2和M4)与人GLP-1R重组蛋白 ( 货号:13944-H02H**,义翘神州)**的结合亲和力比GLP-1R激动剂(Semaglutide)的高10-30倍。(doi: 10.1016/j.molmet.2023.101762)
用于免疫印迹WB检测
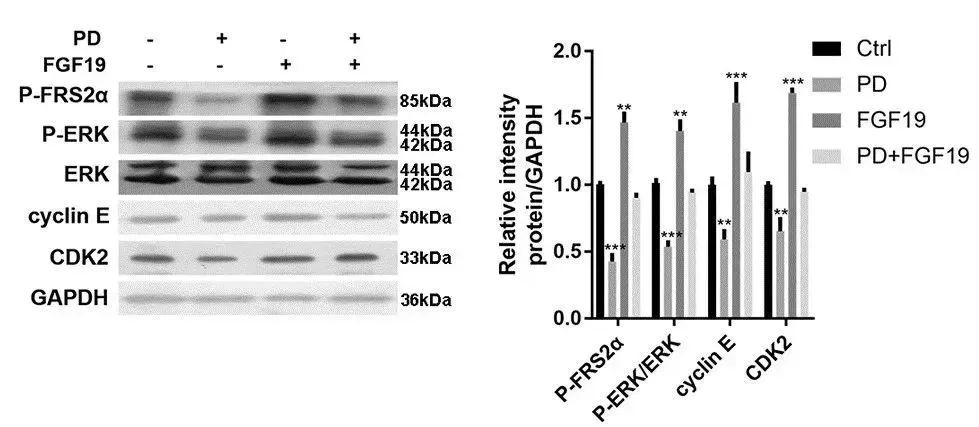
利用FGF19重组蛋白( 货号:12226-HNAE**,义翘神州)**或PD(FGFR4抑制剂)处理HepG2细胞,结果发现FGF19提高P-FRS2α、P-ERK、cyclin E和CDK2表达水平,而PD降低这些蛋白的表达,表明PD能阻断FGFR介导的FRS2α-ERK信号通路,影响肝细胞癌的增殖。(doi: 10.1371/journal.pone.0234708)
用于细胞增殖和侵袭检测
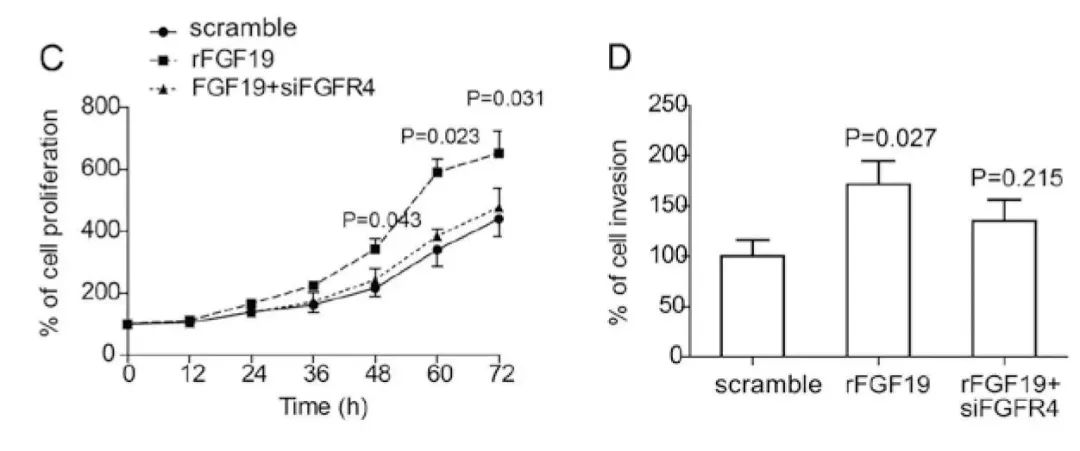
FGF19通过刺激FGFR4促进卵巢癌的进展。(C)**rFGF19重组蛋白(购自义翘神州)**促进卵巢癌OVCAR3细胞增殖,而FGFR4基因敲除会抑制FGF19诱导的OVCAR3细胞增殖。(D)rFGF19重组蛋白诱导的OVCAR3细胞侵袭需要FGFR4。(doi: 10.3892/or.2015.4212)
用于小鼠模型静脉注射检测
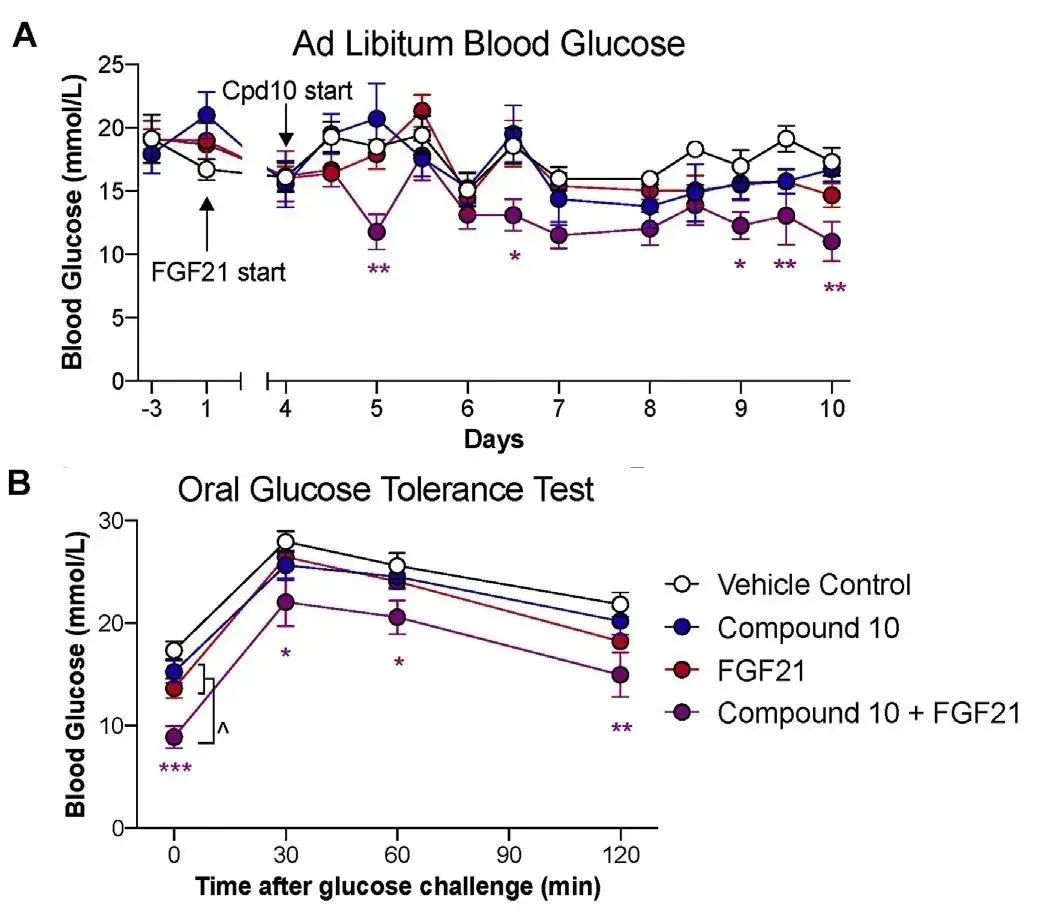
STZ诱导的糖尿病小鼠模型分别接受空白对照组、Compound 10、**FGF21重组蛋白(购自义翘神州)**及联合治疗(Compound 10和FGF21),(A)用药1小时后测量自由血糖浓度,(B)11天后通过口服葡萄糖耐量试验(OGTT)测定血糖水平。(doi: 10.1016/j.molmet.2021.101187.)
✦义翘神州NASH靶点明星产品
高纯度:
THR-β Protein, Human, Recombinant (His Tag), HPLC-verified, Cat: 15737-H07E
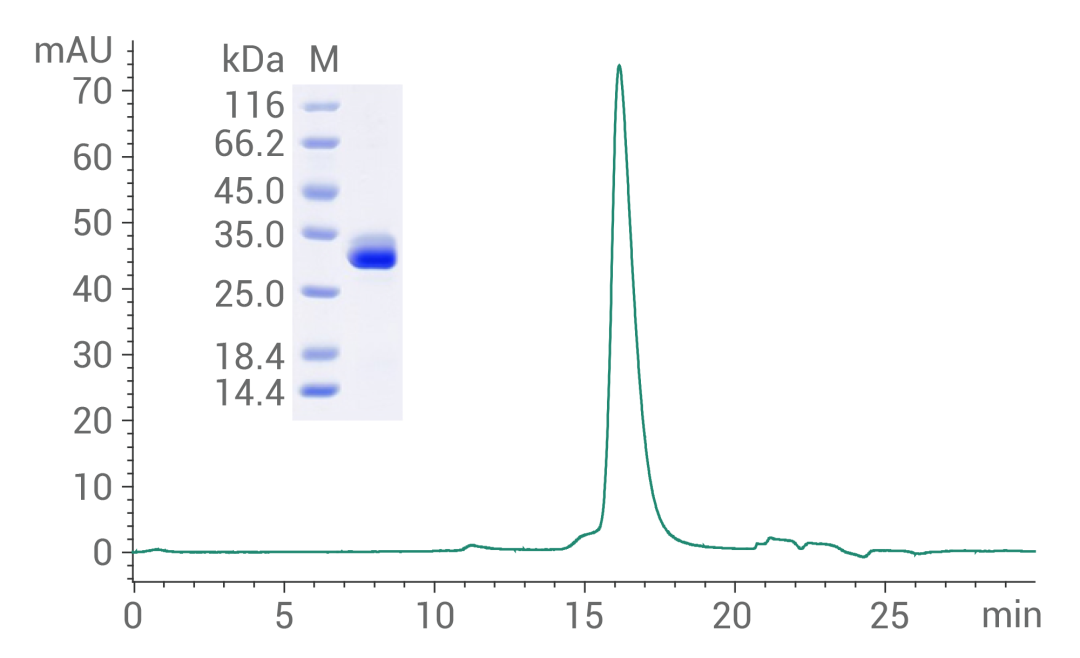
Purity ≥ 95 % as determined by SDS-PAGE,
≥ 90 % as determined by SEC-HPLC.
GLP-1R Protein, Human, Recombinant (His & AVI Tag ), HPLC-verified, Cat: 13944-H49H-B

Purity ≥ 95 % as determined by SDS-PAGE,
≥ 95 % as determined by SEC-HPLC.
|--------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------|--------|---------|-----------|
| 更多NASH靶点明星产品 ||||
| 货号 | 靶点 | 种属 | 纯度/标签 |
| 11769-H07B | ACLY | Human | >85%、His |
| 13944-H02H | GLP-1R | Human | >95%、hFc |
| 30113-H0NASH30113-H0 | FASN | Human | >95%、His |
| 57840-M07E | FASN | Mouse | >95%、His |
| 53744-M07E | NR1H4 | Mouse | >95%、His |
| 12080-H07E | PPARA | Human | >90%、His |
| 56956-M07E | PPARA | Mouse | >90%、His |
| 12226-HNAE | FGF19 | Human | >90% |
| 10911-H07E | FGF21 | Human | >95%、His |
| 50421-M08H | FGF21 | Mouse | >85%、His |
| 10911-HNAE | FGF21 | Human | >95% |
| 5A7643-M08H | FGF21 | Hamster | >95%、His |
注:蛋白纯度均为SDS-PAGE检测结果
【参考文献】
-
Cotter, T. G. & Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 158, 1851--1864 (2020).
-
Kiarash Riazi, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol, 2022. DOI: 10.1016/S2468-1253(22)00165-0
-
NASH Definition & Prevalence - American Liver Foundation. https://liverfoundation.org/liver-diseases/fatty-liver-disease/nonalcoholic-steatohepatitis-nash/nash-definition-prevalence/.
-
van de Wiel, et al. Identification of FDA-approved drugs targeting the Farnesoid X Receptor. Sci Rep 9, (2019).
-
Xu, X. et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduction and Targeted Therapy vol. 7 Preprint at https://doi.org/10.1038/s41392-022-01119-3 (2022).
-
Sanyal, A. J. et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med 29, 392--400 (2023).
-
Feng, X., et al. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog Lipid Res 77, 101006 (2020).
-
Henriksson, E. & Andersen, B. FGF19 and FGF21 for the Treatment of NASH---Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human. Frontiers in Endocrinology vol. 11 Preprint at https://doi.org/10.3389/fendo.2020.601349 (2020).
-
Morrow, M. R. et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab 34, 919-936.e8 (2022).
-
Saponaro, F., Sestito, S., Runfola, M., Rapposelli, S. & Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Frontiers in Medicine vol. 7 Preprint at https://doi.org/10.3389/fmed.2020.00331 (2020).
-
Newsome, P. N. et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. New England Journal of Medicine 384, 1113--1124 (2021).
-
Francque, S. M. et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. New England Journal of Medicine 385, 1547--1558 (2021).
-
Lee, Y. K. et al. FOXO1 inhibition synergizes with FGF21 to normalize glucose control in diabetic mice. Mol Metab 49, (2021).
-
Hu, L. & Cong, L. Fibroblast growth factor 19 is correlated with an unfavorable prognosis and promotes progression by activating fibroblast growth factor receptor 4 in advanced-stage serous ovarian cancer. Oncol Rep 34, 2683--2691 (2015).
-
NASH drugs race to cross the finish line - Pharmaceutical Technology. https://www.pharmaceutical-technology.com/features/nash-drugs-race-to-cross-the-finish-line/.
-
Jensen-Urstad, A. P. L. & Semenkovich, C. F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1821, 747--753 (2012).
-
Qiao, C. et al. PD173074 blocks G1/S transition via CUL3-mediated ubiquitin protease in HepG2 and Hep3B cells. PLoS One 15, (2020).
-
Girdhar, K. et al. Design, synthesis, and biological evaluation of a small molecule oral agonist of the glucagon-like-peptide-1 receptor. Journal of Biological Chemistry 298, (2022).
-
Guo, W. et al. Discovery of ecnoglutide -- A novel, long-acting, cAMP-biased glucagon-like peptide-1 (GLP-1) analog. Mol Metab 101762 (2023) doi:10.1016/j.molmet.2023.101762.