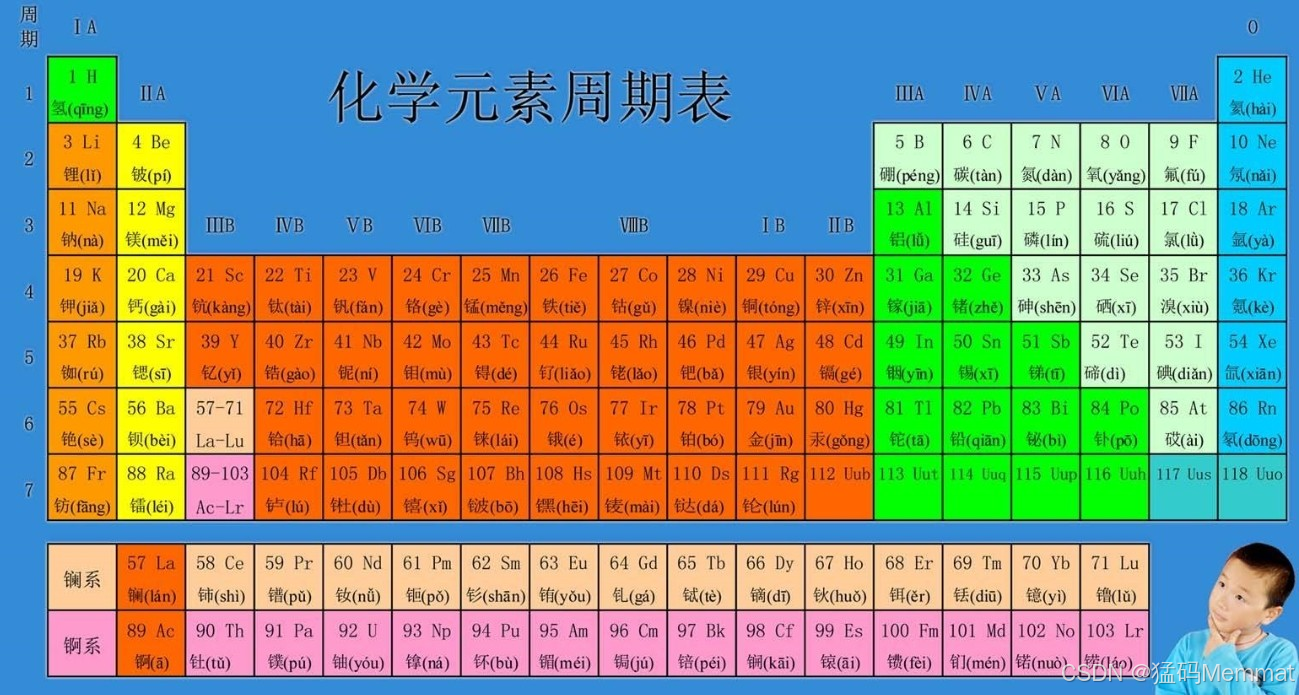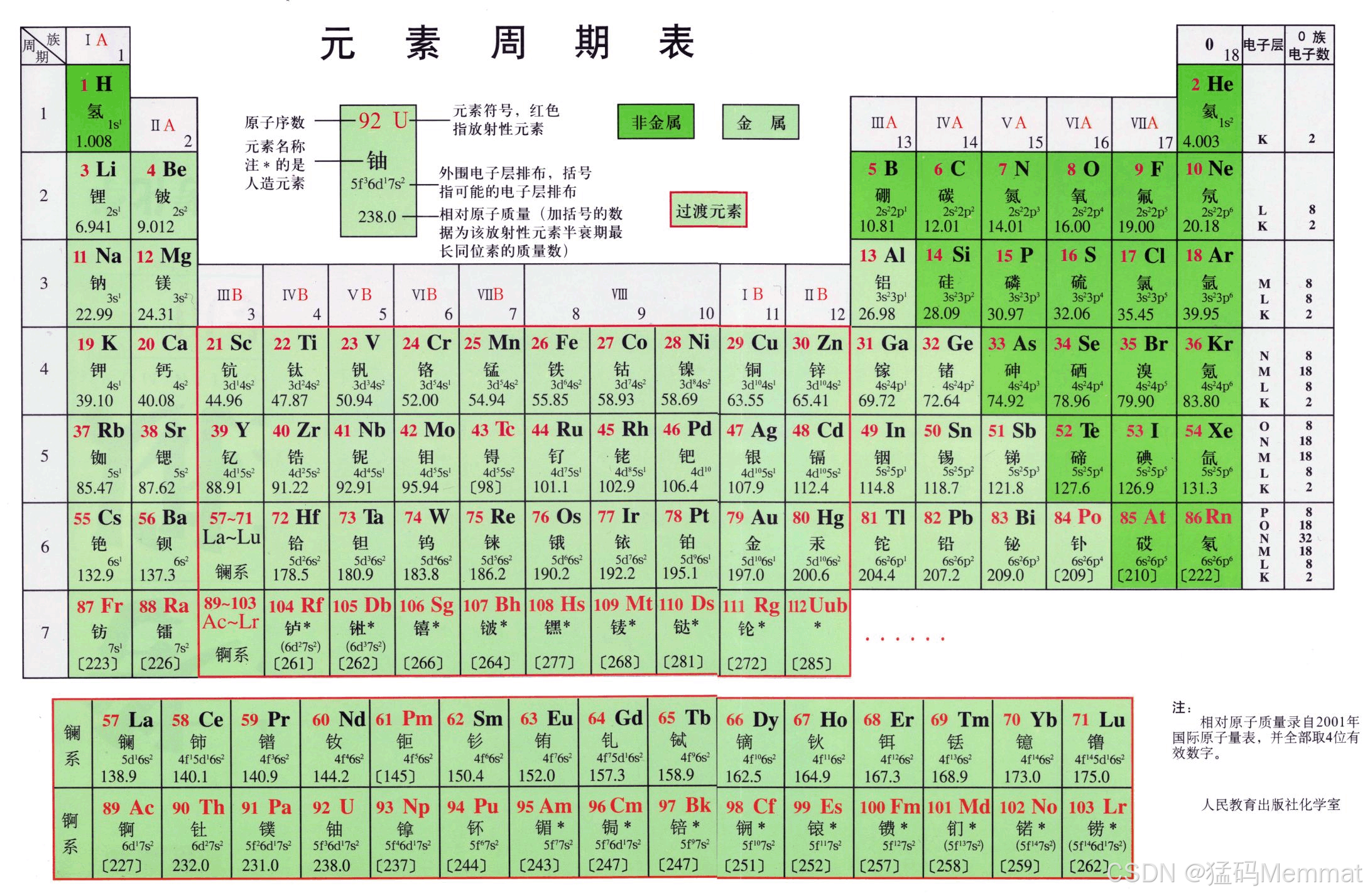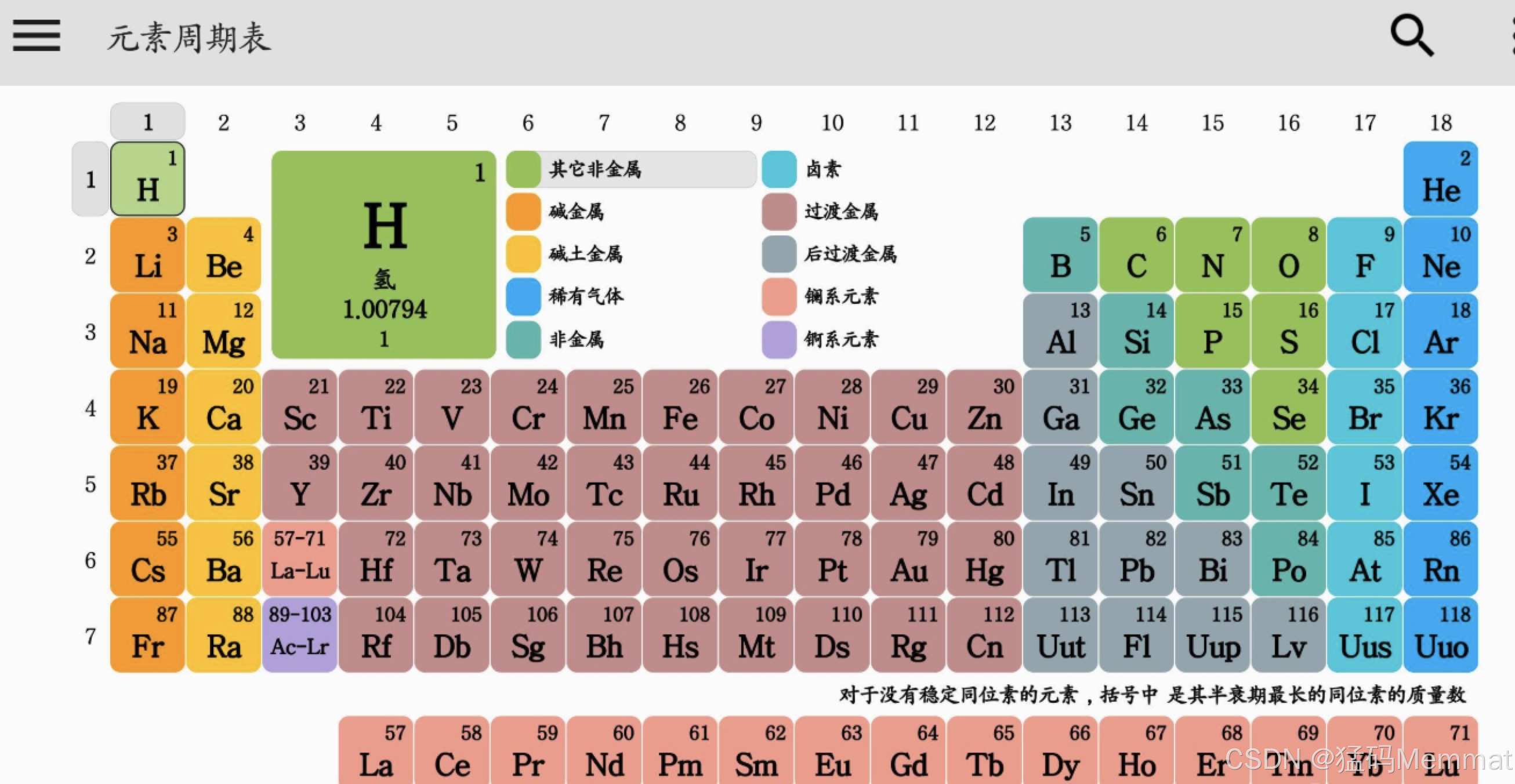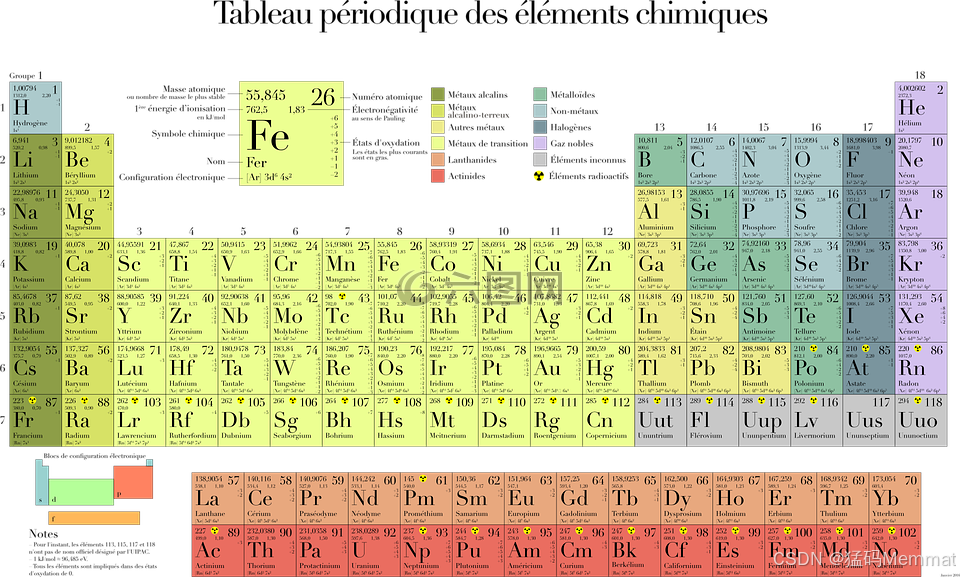
It's not just a chart; it's a map of all the stuff the universe is made of, and the story of how humans learned to read it.
The Chaos Before the Table
For centuries, alchemists and early chemists discovered elements one by one---iron, gold, carbon, mercury. By the early 1800s, dozens were known, but they were just a random list. There was no organization, no sense of how they related. It was like having a box of scattered puzzle pieces with no picture to guide you.
The Dream of Order: Early Attempts
Chemists started noticing patterns. Some elements had similar properties (like chlorine, bromine, and iodine). They tried arranging them by atomic weight (the only number they had). One pioneer, John Newlands, proposed a "Law of Octaves" in 1864, noting properties repeated every eight elements, like musical notes. He was ridiculed; one scientist asked if he'd tried arranging them alphabetically.
The Visionary: Dmitri Mendeleev
Enter Dmitri Mendeleev, a Russian chemistry professor with a problem: he needed a good textbook for his students. In 1869, while writing it, he decided to find a system.
His genius was twofold:
- He left gaps. Mendeleev arranged the known 63 elements by increasing atomic weight and their chemical properties. When the pattern demanded an element that didn't exist, he boldly left a blank space, predicting not only its existence but its properties.
- He broke his own rule when needed. Sometimes, to keep elements with similar properties in the same column, he would swap their order, intuitively sensing something more fundamental than atomic weight was at work. (This would later be understood as atomic number).
His first published table was titled, "An Attempt at a System of Elements, Based on Their Atomic Weight and Chemical Affinity."
The Triumph of Prediction
Mendeleev's predictions seemed like magic. He described three missing elements in detail: "eka-aluminum," "eka-boron," and "eka-silicon."
Within 15 years, they were discovered:
- Gallium (1875) matched eka-aluminum.
- Scandium (1879) matched eka-boron.
- Germanium (1886) matched eka-silicon almost exactly.
This was the moment the scientific world was convinced. Mendeleev wasn't just organizing; he had discovered a natural law.
The Modern Understanding: Why It Works
Mendeleev didn't know why his table worked. The 20th century unlocked the secret: the structure of the atom.
- Atomic Number (Protons): Henry Moseley discovered in 1913 that each element's unique identity is defined by the number of protons in its nucleus. This became the organizing principle, fixing the slight glitches in Mendeleev's weight-based order.
- Electron Shells: Quantum mechanics revealed that elements in the same column (group) have the same number of electrons in their outermost shell. This is why they behave so similarly chemically. The rows (periods) represent filling up those electron shells.
The table's layout is now a map of the atom's structure: reading left to right, you add one proton and one electron. The "shape" of the table---the long blocks---mirrors how electrons fill orbitals (s, p, d, f).
The Living Table Today
Mendeleev's table had empty spaces for new elements. Today, the table is complete through period 7 . All elements up to Oganesson (element 118) have been synthesized, mostly in labs by smashing atoms together. These heavy, unstable elements at the very bottom only exist for fractions of a second, pushing the boundaries of matter.
The story continues in labs where scientists dream of an "island of stability"---theorized superheavy elements that might last longer---and in stars, where every element (except the very heaviest) is forged in nuclear furnaces, reminding us that we are literally made of stardust, cataloged on a single, elegant chart.
In essence, the story is this: From a list of seemingly disconnected substances, through the insight of a man who saw a hidden pattern, to the profound understanding of atomic architecture, the Periodic Table became more than a tool. It is the Rosetta Stone of Chemistry, a profound human achievement that organizes the very building blocks of reality.